pH value
The pH value, according to DIN 38404 (Part 5, 2009 edition), is the negative decimal logarithm of the effective hydrogen-ion activity expressed in mol/l (concentration). It indicates the strength of the acidic or alkaline effect of an aqueous solution.
The scale for determining the pH value extends from 0 to 14. See Fig. 1 pH value
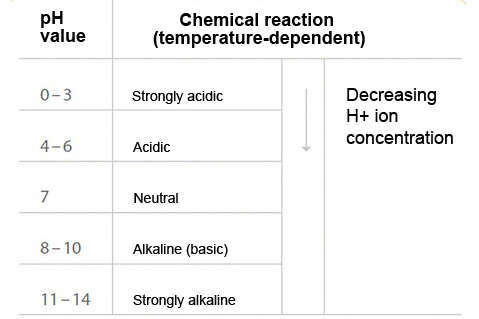
Fig. 1 pH value: Scale for determining the pH value
The pH value can be determined by various methods. The colorimetric analysis method uses indicators which show colour changes at certain pH values (titration), or - in the form of universal indicators (paper, strips etc.) - can be compared with appropriate colour comparison scales.
pH values can be measured with a particularly high degree of accuracy by the electronic method of determining pH value using electrodes (e.g. glass electrodes) and comparison solutions (buffer solutions), see also DIN 19260 and 19261.
